

The cookies is used to store the user consent for the cookies in the category "Necessary". This cookie is set by GDPR Cookie Consent plugin. The cookie is set by GDPR cookie consent to record the user consent for the cookies in the category "Functional".

The cookie is used to store the user consent for the cookies in the category "Analytics". These cookies ensure basic functionalities and security features of the website, anonymously. Necessary cookies are absolutely essential for the website to function properly. Arthur Schuster and William Crooks proved that cathode rays are deflected by electric and magnetic fields, respectively. How are cathode rays deflected in Crookes tube?Ĭrookes tubes are partially vacuum tubes having two electrodes kept at a high potential difference to discharge cathode rays, from the negatively charged electrode, cathode. The pair, in turn, was connected to an electrometer, a device for catching and measuring electric charges. Thomson took a cathode ray tube and at the place where the electron beam was supposed to strike, he positioned a pair of metal cylinders having slits on them. What did Thomson do with the cathode ray tube? A cathode-ray tube (CRT) is a vacuum tube containing one or more electron guns, the beams of which are manipulated to display images on a phosphorescent screen. The only visible differences are the single electron gun, the uniform white phosphor coating, and the lack of a shadow mask.
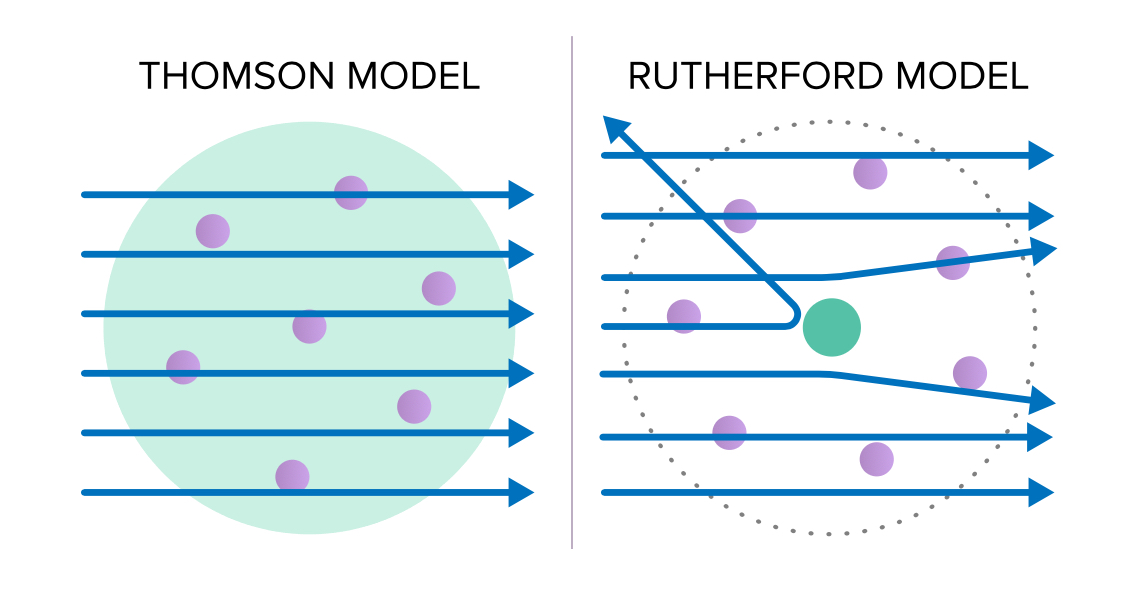
What’s the difference between a cathode ray tube and a CRT? It modulates, accelerates, and deflects electron beam (s) onto the screen to create the images. The cathode-ray tube ( CRT) is a vacuum tube that contains one or more electron guns and a phosphorescent screen and is used to display images. Thomson proposed the plum pudding model of the atom, which had negatively-charged electrons embedded within a positively-charged “soup.” What is the function of a cathode ray tube? Thomson’s experiments with cathode ray tubes showed that all atoms contain tiny negatively charged subatomic particles or electrons. What did the cathode ray tube experiment demonstrate? The images may represent electrical waveforms (oscilloscope), pictures (television set, computer monitor), radar targets, or other phenomena. Other displays technologies are available now which are light weighted, flat, and much more advanced.Ī cathode-ray tube (CRT) is a vacuum tube containing one or more electron guns, the beams of which are manipulated to display images on a phosphorescent screen. Which among the following is a disadvantage of the cathode ray tube?Įxplanation: Being large, heavy, and bulgy cannot be taken as an advantage of the cathode ray tube. Benefits include high-quality, true color grayscale and color imaging, brighter screens, long service life, low heat emissions, less energy consumption (between 30 to 50 percent less than CRT), and most don’t have fans. LCDs offer benefits over traditional cathode ray tube (CRT) monitors and plasma screens. What are the advantages of LCDs over CRTs cathode ray tubes )?
THOMSON CATHODE RAY EXPERIMENT PROVED THAT REGISTRATION
Imperfect focus and color registration also reduce sharpness. The CRT’s Gaussian beam profile produces images with softer edges that are not as sharp as an LCD at its native resolution. What is the advantage and disadvantage cathode ray tube?ĬRTs are less expensive than comparable displays using other display technologies. 5 How are cathode rays deflected in Crookes tube?.4 What is the function of a cathode ray tube?.3 What did the cathode ray tube experiment demonstrate?.


 0 kommentar(er)
0 kommentar(er)
